Retina
Finding an AMD Holy Grail
Ongoing European study searches for intermediate AMD markers.

Gearoid Tuohy
Published: Wednesday, February 1, 2023
By Dr Gearóid Tuohy
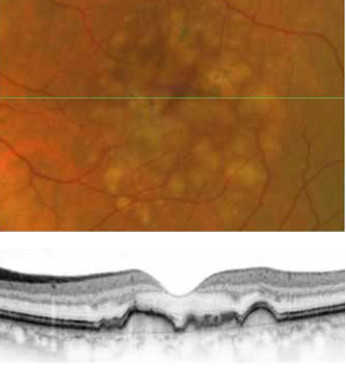
With 700 patients enrolled and well underway, the European MACUSTAR project aims to develop novel clinical endpoints for those with intermediate age-related macular degeneration (iAMD).
There is a significant unmet need for treatment options in both early and intermediate AMD. While there are some valuable treatments for late-stage non-neovascular AMD (i.e., geographic atrophy)—using particular agents to slow the progression of the disease—the “holy grail” for a therapy in the ophthalmic community is a process to intervene earlier in the pathology, preventing the later stages of the disease and, thus, minimising visual loss.
The MACUSTAR project will characterise the functional deficits in iAMD and develop and validate functional, structural, and patient-reported outcome measures. Additional goals include investigating risk factors for progression from iAMD to late AMD, according to Frank Holz MD.
As there may be no universally accepted clinical endpoints for early or intermediate stages of the disease, Professor Holz commented that accomplishing this goal might be challenging. Designing, using, and measuring the “right endpoints,” therefore, will be imperative for MACUSTAR.
“Measuring a patient with intermediate AMD with BCVA would not be helpful, as it may maintain for a long period until it crashes into late-stage disease development,” he explained. “So we need other endpoints accepted by the regulatory authorities to test interventions in these earlier stages.”
MACUSTAR investigators across Europe are assessing methods for structural, functional, and patient-reported outcome tests; reliable test/retest processes; and how the community can differentiate between different neighbouring disease states and how these may change over time.
The project covers this gap in the research work to develop key outcomes supported by expert academic, clinical, and commercial organisations. MACUSTAR will follow more than 700 AMD patients over three years, using state-of-theart imaging techniques, vision testing, and patient-reported outcome measures that capture the disease’s impact on quality of life.
The continent-wide project has 20 clinical sites to date. Each site collects data including mean and point-wise retinal sensitivities on scotopic/mesopic fundus-controlled perimetry, low-luminance visual acuity (LLVA), low luminance deficit, Moorfields Acuity Chart visual acuity, dark adaptation absolute threshold and rod intercept time, and several more assays. Sites also collect patient-reported low-luminance functioning.
One of MACUSTAR’s valuable research outcomes so far is further validation of the “Vision Impairment in Low Luminance (VILL) questionnaire”, which captures visual functioning and vision-related quality of life (VRQoL) under low luminance, low-contrast conditions relevant to AMD.
The relevance of this work only increases as other existing patient-reported outcome instruments do not fulfil regulator development requirements or sufficiently capture AMD patients’ difficulties. The new VILL questionnaire now supports the psychometric performance of patients, including internal consistency, item fit, subscale structure, test/retest reliability, and construct validity in a multinational, multilanguage setting. Prof Holz noted this research could impact clinical research, practice, and policy in the future.
The Innovative Medicines Initiative (IMI), with support from the European Commission and the European Federation of Pharmaceutical Industries and Associations (EFPIA), funds the €16.2M project—scheduled to complete in spring 2024 after running for approximately 6.5 years.
For more information on MACUSTAR, please visit www.macustar.eu
Prof Holz spoke at the 22nd EURETINA Congress in Hamburg.
Frank G Holz MD is Chair of the Department of Ophthalmology at University Hospital Bonn, Germany. frank.holz@ukb.uni-bonn.de
Tags: AMD
Latest Articles
Towards a Unified IOL Classification
The new IOL functional classification needs a strong and unified effort from surgeons, societies, and industry.
The 5 Ws of Post-Presbyopic IOL Enhancement
Fine-tuning refractive outcomes to meet patient expectations.
AI Shows Promise for Meibography Grading
Study demonstrates accuracy in detecting abnormalities and subtle changes in meibomian glands.
Are There Differences Between Male and Female Eyes?
TOGA Session panel underlined the need for more studies on gender differences.
Simulating Laser Vision Correction Outcomes
Individualised planning models could reduce ectasia risk and improve outcomes.
Need to Know: Aberrations, Aberrometry, and Aberropia
Understanding the nomenclature and techniques.
When Is It Time to Remove a Phakic IOL?
Close monitoring of endothelial cell loss in phakic IOL patients and timely explantation may avoid surgical complications.
Delivering Uncompromising Cataract Care
Expert panel considers tips and tricks for cataracts and compromised corneas.
Organising for Success
Professional and personal goals drive practice ownership and operational choices.